linkedin post 2021-05-01 05:44:38
FRAGMENT FROM NATURE for this and next weekend is a paper entitled Sweet taste of heavy water, by lead author Natalie Ben Abu, from the laboratory of Pavel Jungwirth, at the Institute of Organic Chemistry and Biochemistry (IOCB), Czech Academy of Sciences, in Prague. The IOCB is one of the 54 institutes in The Czech Academy of Sciences of the Czech Republic, the institution which primary mission is to conduct basic research in a broad spectrum of the natural, technical and social sciences and the humanities. https://lnkd.in/d85eSEg View in LinkedIn
linkedin post 2021-05-01 05:46:17
IOCB is 50% funded by $90 million/year in royalties from 15 innovative drugs that it has licensed, including : Antivirotics: Duviragel™, cidofovir (Vistide™), adefovir (Hepsera™), tenofovir (Viread™, Truvada™, Atripla™, Striblid™, Complera™); Peptides: Oxytocin™, Adiuretin™, Terlipressin™; Natural Substances: Dermazulen, Lafarex®.” (IOCB = Institute of Organic Chemistry and Biochemistry). https://lnkd.in/d85eSEg View in LinkedIn
linkedin post 2021-05-01 05:48:29
HEAVY WATER. “The most notable difference in physical properties between D2O and H2O is the roughly 10% higher density of the former liquid, which is mostly a trivial consequence of deuterium being about twice as heavy as hydrogen.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

linkedin post 2021-05-01 05:49:07
PHYSICAL PROPERTIES. “A more subtle effect of deuteration is the formation of slightly stronger hydrogen (or deuterium) bonds in D2O as compared to H2O3. This results in a small increase of the freezing and boiling points by 3.8 °C and 1.4 °C, respectively, and in a slight increase of 0.44 in pH (or pD) of pure water upon deuteration.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

linkedin post 2021-05-01 05:50:54
D2O. “In comparison, a mere dissolution of atmospheric CO2 and subsequent formation of dilute carbonic acid in open containers has a significantly stronger influence on the pH of water, changing it by more than one unit.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

linkedin post 2021-05-01 05:51:27
“BIOLOGICAL EFFECTS are observable for high doses of D2O. While bacteria or yeasts can function in practically pure D2O, albeit with somewhat hindered growth rate, for higher organisms damaging effects on cell division and general metabolism occur at around 25% deuteration, with lethal conditions for plants and animals typically occurring at ~40–50% deuteration of the body water.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

linkedin post 2021-05-01 05:53:35
TOXICOLOGY. “Small levels of deuteration are, nevertheless, harmless. This is understandable given the fact that about 1 in every 6400 hydrogens in nature is found in its stable isotope form of deuterium. Oral doses of several milliliters of D2O are safe for humans and are used in the isotopic form D218O for metabolic measurements in clinical praxis (known as “doubly labeled water” technique).” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

linkedin post 2021-05-01 05:54:42
CIRCADIAN EFFECT. “Probably the best-established effect of D2O is the increase of the circadian oscillation length upon its administration to both animals and plants. This has been attributed to a general slowdown of metabolism upon deuteration, although the exact mechanism of this effect is unknown.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn

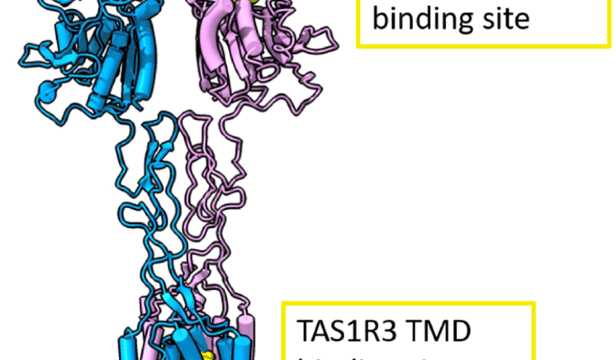
linkedin post 2021-05-01 05:56:26
SWEETENERS. “Interestingly, not all artificial sweeteners are recognized by rodents. Differences in human and rodent responses to tastants, as well as sweetness inhibitors such as lactisole, have been useful for delineating the molecular recognition of sweet compounds—using human-mouse chimeric receptors, it was shown that the transmembrane domain (TMD) of human TAS1R3 is required for the activating effects of cyclamate29 and for the inhibitory effect of lactisole.” https://www.nature.com/articles/s42003-021-01964-y View in LinkedIn
